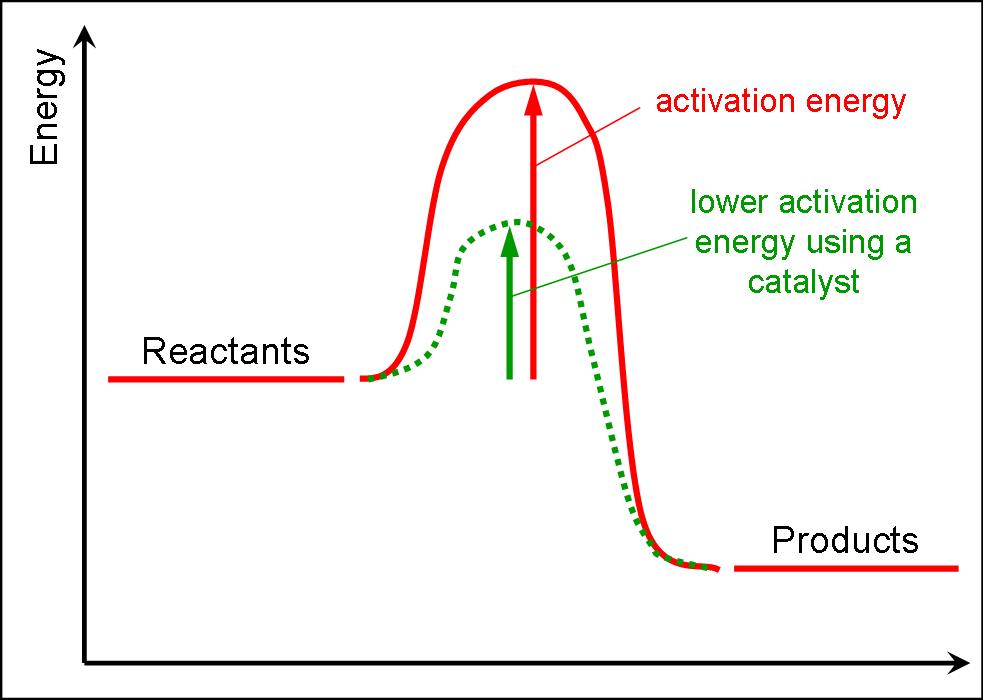
Does Adding A Catalyst Lower Activation Energy . It does not lower the activation energy of the reaction. catalyst lower activation energy is: A catalyst provides an alternative route for the. adding a catalyst has exactly this effect of shifting the activation energy. this does not change the frequency of collisions. — a catalyst provides an alternative route for the reaction with a lower activation energy. By altering the reaction's transition state, a catalyst lowers the activation energy. However, it does increase the frequency of successful collisions. — catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. catalysts decrease the activation energy required for a reaction to proceed (shown by the smaller magnitude of the activation energy on the energy.
from as-bio-and-chem.blogspot.com
By altering the reaction's transition state, a catalyst lowers the activation energy. catalysts decrease the activation energy required for a reaction to proceed (shown by the smaller magnitude of the activation energy on the energy. — catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. adding a catalyst has exactly this effect of shifting the activation energy. However, it does increase the frequency of successful collisions. catalyst lower activation energy is: A catalyst provides an alternative route for the. this does not change the frequency of collisions. — a catalyst provides an alternative route for the reaction with a lower activation energy. It does not lower the activation energy of the reaction.
Bio+Chem Notes. ^^ Recapping Rates of Reaction
Does Adding A Catalyst Lower Activation Energy adding a catalyst has exactly this effect of shifting the activation energy. A catalyst provides an alternative route for the. However, it does increase the frequency of successful collisions. — catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. this does not change the frequency of collisions. — a catalyst provides an alternative route for the reaction with a lower activation energy. It does not lower the activation energy of the reaction. By altering the reaction's transition state, a catalyst lowers the activation energy. adding a catalyst has exactly this effect of shifting the activation energy. catalysts decrease the activation energy required for a reaction to proceed (shown by the smaller magnitude of the activation energy on the energy. catalyst lower activation energy is:
From nesslabs.com
Activation energy the chemistry of getting started Ness Labs Does Adding A Catalyst Lower Activation Energy catalysts decrease the activation energy required for a reaction to proceed (shown by the smaller magnitude of the activation energy on the energy. this does not change the frequency of collisions. By altering the reaction's transition state, a catalyst lowers the activation energy. catalyst lower activation energy is: adding a catalyst has exactly this effect of. Does Adding A Catalyst Lower Activation Energy.
From www.sliderbase.com
Catalysis Presentation Chemistry Does Adding A Catalyst Lower Activation Energy By altering the reaction's transition state, a catalyst lowers the activation energy. — a catalyst provides an alternative route for the reaction with a lower activation energy. this does not change the frequency of collisions. catalyst lower activation energy is: However, it does increase the frequency of successful collisions. adding a catalyst has exactly this effect. Does Adding A Catalyst Lower Activation Energy.
From www.slideserve.com
PPT Reaction Rates PowerPoint Presentation, free download ID6614655 Does Adding A Catalyst Lower Activation Energy It does not lower the activation energy of the reaction. By altering the reaction's transition state, a catalyst lowers the activation energy. adding a catalyst has exactly this effect of shifting the activation energy. catalysts decrease the activation energy required for a reaction to proceed (shown by the smaller magnitude of the activation energy on the energy. A. Does Adding A Catalyst Lower Activation Energy.
From chem.libretexts.org
12.2 Catalytic Hydrogenation Chemistry LibreTexts Does Adding A Catalyst Lower Activation Energy catalysts decrease the activation energy required for a reaction to proceed (shown by the smaller magnitude of the activation energy on the energy. this does not change the frequency of collisions. By altering the reaction's transition state, a catalyst lowers the activation energy. catalyst lower activation energy is: However, it does increase the frequency of successful collisions.. Does Adding A Catalyst Lower Activation Energy.
From 162.254.255.22
When Less Is More Energy Frontier Research Centers Community site Does Adding A Catalyst Lower Activation Energy By altering the reaction's transition state, a catalyst lowers the activation energy. It does not lower the activation energy of the reaction. adding a catalyst has exactly this effect of shifting the activation energy. catalysts decrease the activation energy required for a reaction to proceed (shown by the smaller magnitude of the activation energy on the energy. A. Does Adding A Catalyst Lower Activation Energy.
From www.slideserve.com
PPT Reaction Rates (Chapter 13) PowerPoint Presentation, free Does Adding A Catalyst Lower Activation Energy — a catalyst provides an alternative route for the reaction with a lower activation energy. this does not change the frequency of collisions. However, it does increase the frequency of successful collisions. catalysts decrease the activation energy required for a reaction to proceed (shown by the smaller magnitude of the activation energy on the energy. —. Does Adding A Catalyst Lower Activation Energy.
From kenya-khurst.blogspot.com
Catalysts Lower the Activation Energy of a Reaction by Does Adding A Catalyst Lower Activation Energy adding a catalyst has exactly this effect of shifting the activation energy. — a catalyst provides an alternative route for the reaction with a lower activation energy. catalysts decrease the activation energy required for a reaction to proceed (shown by the smaller magnitude of the activation energy on the energy. It does not lower the activation energy. Does Adding A Catalyst Lower Activation Energy.
From www.labunlimited.com
Solid Phase Catalysis in Continuous Flow Chemistry Lab Unlimited Does Adding A Catalyst Lower Activation Energy catalysts decrease the activation energy required for a reaction to proceed (shown by the smaller magnitude of the activation energy on the energy. — a catalyst provides an alternative route for the reaction with a lower activation energy. However, it does increase the frequency of successful collisions. By altering the reaction's transition state, a catalyst lowers the activation. Does Adding A Catalyst Lower Activation Energy.
From byjus.com
How does a catalyst increase the rate of a reaction? Does Adding A Catalyst Lower Activation Energy catalysts decrease the activation energy required for a reaction to proceed (shown by the smaller magnitude of the activation energy on the energy. A catalyst provides an alternative route for the. this does not change the frequency of collisions. adding a catalyst has exactly this effect of shifting the activation energy. — a catalyst provides an. Does Adding A Catalyst Lower Activation Energy.
From as-bio-and-chem.blogspot.com
Bio+Chem Notes. ^^ Recapping Rates of Reaction Does Adding A Catalyst Lower Activation Energy — catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. catalysts decrease the activation energy required for a reaction to proceed (shown by the smaller magnitude of the activation energy on the energy. this does not change the frequency of collisions. A catalyst provides an alternative route for the.. Does Adding A Catalyst Lower Activation Energy.
From www.slideserve.com
PPT Mechanisms of Catalytic Reactions and Characterization of Does Adding A Catalyst Lower Activation Energy adding a catalyst has exactly this effect of shifting the activation energy. By altering the reaction's transition state, a catalyst lowers the activation energy. — a catalyst provides an alternative route for the reaction with a lower activation energy. A catalyst provides an alternative route for the. However, it does increase the frequency of successful collisions. catalyst. Does Adding A Catalyst Lower Activation Energy.
From dxopopljn.blob.core.windows.net
Enzyme Catalyzed Reactions at Richard Fountain blog Does Adding A Catalyst Lower Activation Energy A catalyst provides an alternative route for the. catalysts decrease the activation energy required for a reaction to proceed (shown by the smaller magnitude of the activation energy on the energy. It does not lower the activation energy of the reaction. — a catalyst provides an alternative route for the reaction with a lower activation energy. By altering. Does Adding A Catalyst Lower Activation Energy.
From 2012books.lardbucket.org
Catalysis Does Adding A Catalyst Lower Activation Energy A catalyst provides an alternative route for the. this does not change the frequency of collisions. catalyst lower activation energy is: — catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. However, it does increase the frequency of successful collisions. By altering the reaction's transition state, a catalyst lowers. Does Adding A Catalyst Lower Activation Energy.
From www.thoughtco.com
Catalysis Definition in Chemistry Does Adding A Catalyst Lower Activation Energy A catalyst provides an alternative route for the. catalysts decrease the activation energy required for a reaction to proceed (shown by the smaller magnitude of the activation energy on the energy. adding a catalyst has exactly this effect of shifting the activation energy. However, it does increase the frequency of successful collisions. — a catalyst provides an. Does Adding A Catalyst Lower Activation Energy.
From www.cheric.org
Chemical Reaction (Reaction rate) Does Adding A Catalyst Lower Activation Energy — a catalyst provides an alternative route for the reaction with a lower activation energy. A catalyst provides an alternative route for the. catalysts decrease the activation energy required for a reaction to proceed (shown by the smaller magnitude of the activation energy on the energy. adding a catalyst has exactly this effect of shifting the activation. Does Adding A Catalyst Lower Activation Energy.
From dxooagcgl.blob.core.windows.net
How Does The Presence Of A Catalyst Affect The Activation Energy Of A Does Adding A Catalyst Lower Activation Energy It does not lower the activation energy of the reaction. catalysts decrease the activation energy required for a reaction to proceed (shown by the smaller magnitude of the activation energy on the energy. However, it does increase the frequency of successful collisions. A catalyst provides an alternative route for the. this does not change the frequency of collisions.. Does Adding A Catalyst Lower Activation Energy.
From wou.edu
Chapter 7 Catalytic Mechanisms of Enzymes Chemistry Does Adding A Catalyst Lower Activation Energy this does not change the frequency of collisions. — a catalyst provides an alternative route for the reaction with a lower activation energy. — catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. catalyst lower activation energy is: It does not lower the activation energy of the reaction.. Does Adding A Catalyst Lower Activation Energy.
From www.slideserve.com
PPT Enzyme Biological Catalyst (Part ii) PowerPoint Presentation Does Adding A Catalyst Lower Activation Energy It does not lower the activation energy of the reaction. this does not change the frequency of collisions. However, it does increase the frequency of successful collisions. catalysts decrease the activation energy required for a reaction to proceed (shown by the smaller magnitude of the activation energy on the energy. By altering the reaction's transition state, a catalyst. Does Adding A Catalyst Lower Activation Energy.